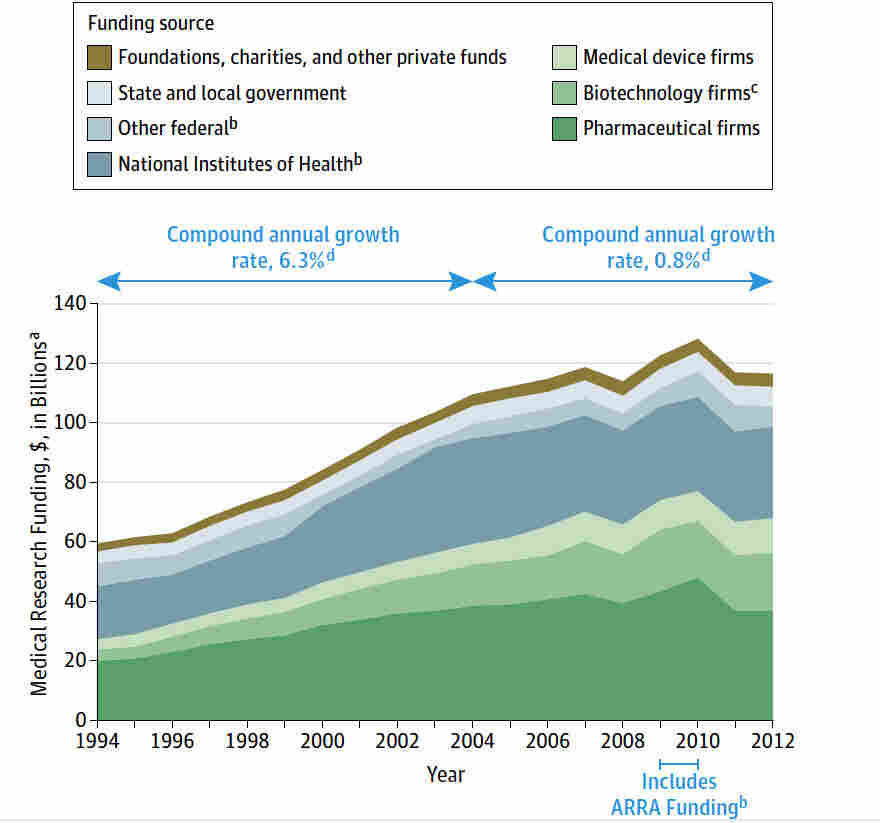
Medical research funding plays a critical role in advancing healthcare research, enabling scientific breakthroughs that enhance patient care and treatment options. Without adequate financial support from institutions like the National Institutes of Health, vital studies face significant disruptions, jeopardizing the safety of participants involved in clinical trials. The halt in grants can severely compromise the oversight provided by institutional review boards (IRBs), which are essential for ensuring compliance with ethical standards and regulatory requirements. Further, funding cuts impact the collaborative efforts necessary for effective clinical trial safety, hindering the ability to monitor and protect research subjects effectively. As we delve deeper into the implications of these funding challenges, we must recognize their far-reaching consequences on both research integrity and public health.
The financial backing of health investigations is paramount to fostering innovation and securing the safety of individuals enrolled in medical studies. When governmental funding for healthcare experimentation is constrained, researchers across various institutions, including hospitals and universities, find themselves unable to pursue groundbreaking projects. This financial strain can hinder the operational processes of institutional review boards, which oversee participant safety and ensure ethical standards are upheld in trials. Moreover, when research funding diminishes, it stifles collaboration across sites, which is crucial for evaluating new treatments and therapies. It is vital that we address the repercussions of funding shortages to maintain trust in the medical research agenda and protect the welfare of study participants.
The Importance of Medical Research Funding
Medical research funding plays a vital role in advancing healthcare and ensuring patient safety. With significant financial resources, researchers can conduct critical studies that lead to new treatments, enhance clinical trial safety, and innovate healthcare practices. Without adequate funding from organizations like the National Institutes of Health (NIH), vital research initiatives faced with funding cuts would struggle to sustain ongoing projects, affecting not only the scientific community but also patients who rely on these advancements for better health outcomes.
These funds are used to support rigorous oversight of research activities, ensuring compliance with institutional review board (IRB) requirements. A well-funded research environment enables institutions to recruit top talent, maintain ethical standards, and invest in cutting-edge technologies to enhance research methodologies. The halt in funding disrupts these essential functions, placing the safety of patients participating in trials at risk and impeding the progress of medical breakthroughs.
Impact of Funding Cuts on Clinical Trials
The halt in medical research funding has far-reaching consequences for clinical trials, as it directly affects their initiation and execution. When funding is cut, ongoing trials may face significant interruptions, delaying critical research outputs and the potential introduction of new therapies to the market. This situation detrimentally impacts patients who are often eager to participate in clinical trials that could offer them hope for effective treatments.
Moreover, many research institutions face challenges in maintaining the rigorous IRB oversight required to protect trial participants. A robust IRB system ensures that ethical guidelines are followed, risks are minimized, and the safety of participants is prioritized. Funding cuts threaten not only the continuity of ongoing clinical trials but also the integrity of the research process itself, leading to widespread skepticism and potential harm to the reputations of both researchers and institutions.
The Role of IRB Oversight in Patient Safety
Institutional Review Boards (IRBs) are critical to safeguarding the rights and welfare of individuals involved in medical research. Their primary function is to review studies to ensure that they comply with ethical standards and regulations established by local, state, and federal guidelines. IRBs are responsible for monitoring patient safety, assessing risks, and ensuring that informed consent processes are followed diligently.
Without proper funding, the effectiveness of these oversight bodies may be compromised. Funding cuts can lead to reductions in the number of qualified personnel dedicated to IRB oversight, resulting in slower review processes and potential lapses in ethical scrutiny. The consequences of insufficient oversight can jeopardize patient safety and diminish public trust in research initiatives, which are essential for advancing health science.
Adapting to Research Funding Challenges
In the face of funding cuts, research institutions must adapt to ensure the continuity of critical projects. This includes exploring alternative funding sources, such as private foundations or partnerships with pharmaceutical companies, to alleviate the impact of federal grant reductions. Collaboration across institutions can also optimize resources and expedite the research process, ensuring that patient safety and innovation remain priorities.
Institutions may also consider streamlining their administrative processes and enhancing engagement with community stakeholders to build trust and transparency. By involving patient representatives in discussions about research priorities, scientists can better align their studies with public health needs, ultimately fostering a more supportive environment for both researchers and participants.
Training and Supporting Research Professionals
With the increasing complexity of medical research, specialized training for research professionals is essential for maintaining high ethical standards and compliance within the field. Ongoing educational programs that focus on the latest ethical guidelines, IRB requirements, and patient safety protocols will ensure that clinical trial staff are well-equipped to navigate challenges even amidst funding disruptions.
Research institutions should invest in developing training resources that empower professionals to understand not just the administrative aspects of research but also ethical considerations. Building a culture of safety within research settings will enhance the protection of trial participants and promote sustainable, ethical research practices, ensuring that impactful medical innovations continue to emerge.
The Future of Healthcare Research
The ongoing financial challenges in the landscape of healthcare research indicate an urgent need for policy reforms that support sustainable funding models. As funding cuts threaten the infrastructure that upholds clinical trial safety and IRB oversight, it is imperative that stakeholders advocate for robust investment in research initiatives, particularly those stemming from the NIH.
The future of healthcare innovations depends on creating an ecosystem where funding is prioritized, simplified processes are in place, and ethical standards are adhered to. Engaging policymakers, educators, and community leaders in discussions about the significance of medical research funding can foster a more supportive environment, ensuring that advancements in health come to fruition despite prevailing challenges.
Building Public Trust in Research
Increasing public trust in scientific research is essential for successful participation in clinical trials. Given past historical injustices in medical research, transparent communication regarding the importance of ethical research oversight, patient rights, and safety measures implemented by IRBs are crucial in rebuilding this trust. Research institutions need to actively engage with communities to dispel myths and educate the public about the significance of their contributions to advancing medical knowledge.
Utilizing social media, community workshops, and public forums can help bridge the gap between researchers and the communities they serve. By prioritizing clear communication and showcasing the benefits of successful research outcomes, institutions can inspire community involvement and shape a more informed and supportive public perspective on clinical research endeavors.
Collaborative Efforts in Medical Research
Collaboration across multiple institutions can enhance the efficiency of medical research while also addressing the issues posed by funding cuts. By engaging in multisite studies, researchers can pool their resources and expertise, thus facilitating more comprehensive and robust research designs. Collaborative networks such as SMART IRB are particularly valuable as they allow for shared oversight and ethical review processes, ensuring participants are better protected across diverse study locations.
Moreover, leveraging collaborative agreements can ensure that even amidst funding cuts, critical research projects continue to thrive. By establishing partnerships with private enterprises, non-profits, and academic institutions, researchers can diversify their funding sources and maintain focus on patient safety and innovative outcomes, securing a brighter future for healthcare research.
Policy Changes Needed for Sustainable Research Funding
There is an urgent need for policies that prioritize sustainable funding for medical research. Policymakers, research institutions, and advocacy groups must collaborate to devise a comprehensive approach that addresses the funding challenges plaguing the medical research landscape. Providing stable and predictable funding streams will empower researchers to design and execute long-term studies that prioritize patient safety and ethical oversight.
Additionally, fostering public-private partnerships can decrease reliance on federal funding alone while facilitating innovation in healthcare research. By embedding accountability and transparency within these funding models, stakeholders can assure participants and the public that their safety and interests continue to remain paramount in clinical research.
Importance of Ethical Framework in Clinical Research
Establishing a strong ethical framework in clinical research is critical in safeguarding patient rights and ensuring their safety. Institutions conducting research must adhere to the highest ethical standards, which include obtaining informed consent, protecting participant privacy, and ensuring appropriate risk assessment. Funding cuts that impact ethical oversight can diminish the commitment of research professionals to uphold ethical norms, ultimately harming patient safety.
Ensuring that all clinical research projects are grounded in ethical principles requires consistent revenue flows towards training and education for research personnel. Additionally, research institutions must adopt clear policies that reinforce ethical practices, thereby safeguarding public trust in the research process while promoting ongoing engagement and support from the communities they serve.
Frequently Asked Questions
How does medical research funding from the NIH affect patient safety in clinical trials?
Medical research funding from the National Institutes of Health (NIH) plays a crucial role in safeguarding patient safety during clinical trials. NIH funds support the implementation and oversight of Institutional Review Boards (IRBs), which ensure that research involving human participants adheres to ethical standards and regulatory requirements. By providing funding for multisite collaborative studies, the NIH enables effective review and oversight, enhancing the protection of patient rights and welfare throughout the research lifecycle.
What is the impact of funding cuts on healthcare research and patient participation?
Funding cuts significantly hinder healthcare research by disrupting ongoing studies, leading to halted projects and reduced patient participation. These cuts can undermine the work of IRBs that are vital in ensuring participant safety, as fewer resources lead to diminished oversight capabilities. The cessation of grants and contracts can discourage researchers from pursuing innovative studies, ultimately affecting the availability of safe and effective treatments for patients.
How do IRB oversight and medical research funding work together to ensure patient safety?
IRB oversight is integral to the ethical management of medical research funded by federal entities such as the NIH. With adequate funding, IRBs can effectively review research proposals, monitor study compliance, and assess risks to participants. This funding supports the necessary training and resources that keep both oversight mechanisms and research practices aligned with ethical standards, thus ensuring the safety and rights of individuals involved in clinical studies.
In what ways do funding cuts impact the ethical considerations in clinical trials?
Funding cuts directly impact ethical considerations in clinical trials by limiting the resources available for IRB oversight and participant protections. Reduced funding may lead to fewer IRB members, insufficient training for oversight committees, and compromised participant safety protocols. This can heighten the risk of ethical breaches and diminish public trust in the research process, potentially deterring individuals from participating in clinical trials.
What measures are in place to protect patients in healthcare research funding initiatives?
Healthcare research funding initiatives typically incorporate robust IRB oversight measures to protect patients. Federal regulations require IRB review for studies involving human participants, ensuring compliance with ethical standards and participant rights. Funding from the NIH supports these necessary oversight functions, allowing researchers to implement the necessary protections and protocols that prioritize the health and safety of study participants throughout the research process.
How does the SMART IRB system enhance medical research funding efficiencies?
The SMART IRB system streamlines the review process for multisite research, thereby enhancing medical research funding efficiencies. By allowing a single IRB to oversee studies conducted across multiple institutions, it reduces administrative burdens and accelerates the initiation of clinical trials. This efficiency ensures that funding can be directed towards patient safety and research integrity rather than entangled in bureaucratic delays, ultimately benefiting all stakeholders involved.
| Key Point | Details |
|---|---|
| Funding Freeze | A halt in funding impacts over $2 billion in federal grants, disrupting medical research efforts. |
| Role of SMART IRB | SMART IRB facilitates oversight for multi-site medical research, ensuring patient rights and safety. |
| Importance of IRBs | IRBs review research proposals, maintain patient safety, and ensure ethical standards in studies. |
| Impact of Funding Cuts | Cuts to funding will halt ongoing studies, increase risks to participants, and damage public trust. |
| Historical Lessons | Past unethical research has led to the establishment of IRBs to safeguard participant welfare. |
Summary
Medical research funding is critical to ensuring the safety and rights of patients involved in clinical studies. The recent freeze on federal grants has raised serious concerns about the ability to maintain oversight and ethical standards in medical research, jeopardizing participant safety and trust in research practices. Supporting robust medical research funding is essential for advancing healthcare and safeguarding participants who contribute to scientific progress.