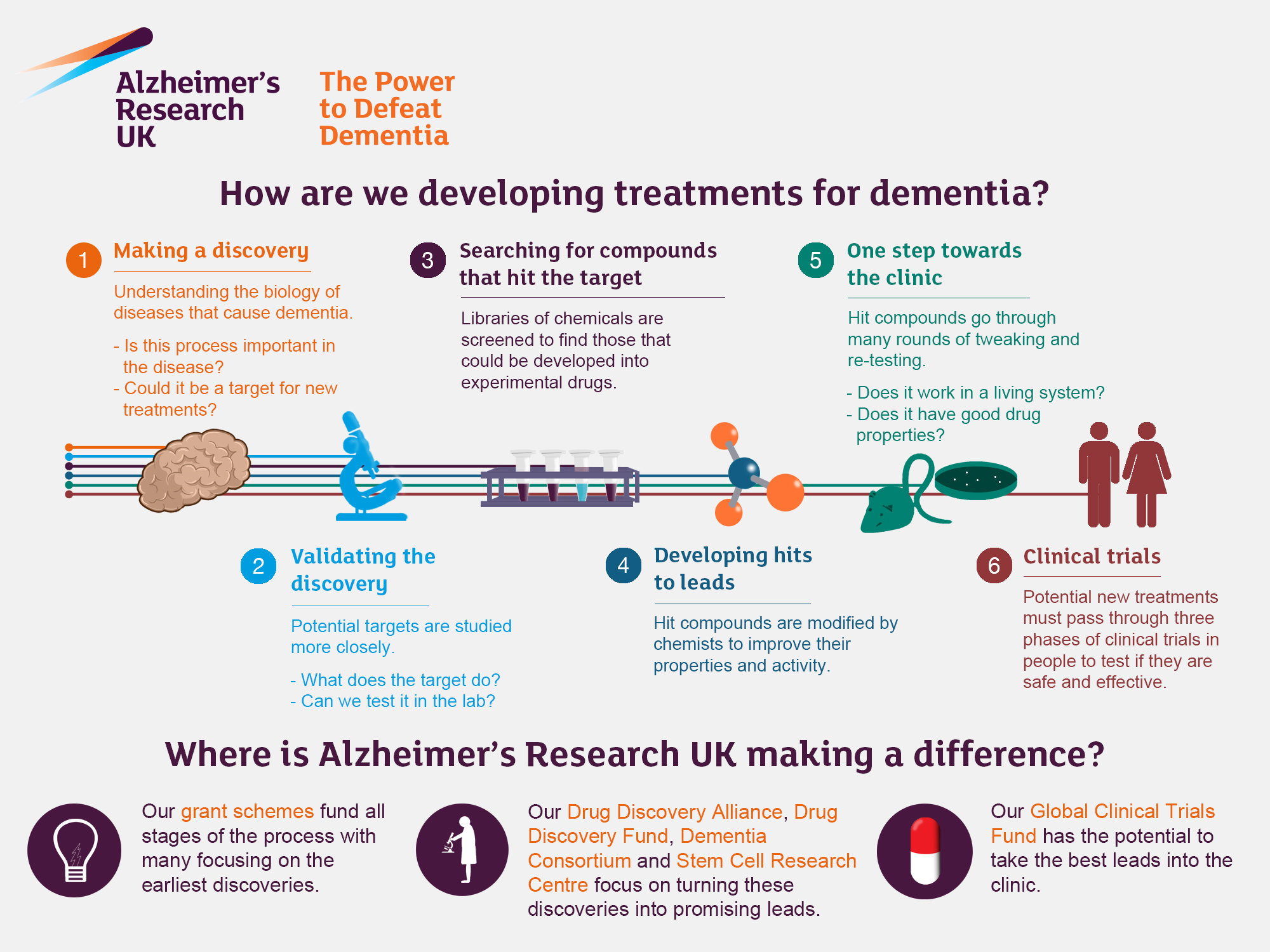
Alzheimer’s research has become a critical focal point as scientists strive to unravel the complexities of this devastating disease. Leading the charge is neuroscientist Beth Stevens, whose groundbreaking studies on microglial cells are reshaping our understanding of the brain’s immune system. These cells play a vital role in maintaining brain health by clearing out dead cells and fine-tuning synaptic connections, but their malfunctioning can contribute to neurodegenerative diseases, including Alzheimer’s. By identifying the intricacies of these immune responses within the brain, her lab is paving the way for innovative Alzheimer’s treatment options. The potential impact of this research cannot be overstated, especially with the rising number of Americans affected by Alzheimer’s projected to double by 2050, highlighting an urgent need for effective therapeutic strategies.
Research focused on Alzheimer’s disease is not just about understanding memory loss; it encompasses a broader investigation into neurodegenerative disorders and the immune mechanisms within the brain. Terms like ‘microglial dysfunction’ and ‘brain immune responses’ are pivotal as they represent a vital link in the chain of discovery leading to potential treatments. The work of scientists like Beth Stevens illuminates the role these cells play in neuroinflammation and synaptic health, thereby offering insights that could transform how we approach diseases such as Alzheimer’s. By investigating the delicate balance of the brain’s immune system, researchers aim to unlock new avenues for prevention and treatment. Overall, the collective efforts in this domain underscore the profound implications of neuroscience on public health and the quality of life for millions.
Understanding Microglial Cells in Alzheimer’s Research
Microglial cells are intrinsic immune cells within the brain, playing a crucial role in maintaining neural health. These cells constantly survey their environment, responding to pathogenic threats and maintaining homeostasis. In the realm of Alzheimer’s research, the functionality and efficiency of microglia are particularly significant. When these cells become activated, they can either support or hinder neuronal survival. This dual role makes them vital in understanding the mechanisms underlying neurodegenerative diseases such as Alzheimer’s, Huntington’s, and others.
Recent investigations led by Beth Stevens have highlighted the importance of microglial pruning in synaptic health. While this process is fundamental during brain development, its dysregulation can lead to the mismanagement of synapses, contributing directly to cognitive decline seen in Alzheimer’s patients. By studying the behavior of microglial cells, Stevens and her team aim to unlock new pathways for intervention, paving the way for innovative treatments that can modulate these immune responses and potentially halt or slow the progression of Alzheimer’s disease.
The Role of the Brain’s Immune System in Neurodegenerative Diseases
The brain’s immune system, primarily orchestrated by microglial cells, represents a frontline defense against neurodegenerative diseases. These cells not only keep the brain clean by phagocytosing debris but also play intricate roles in neuroinflammation, which has been linked to various disorders, including Alzheimer’s and multiple sclerosis. In Stevens’ research, the delicate balance maintained by microglia is emphasized, as overactive or underactive microglial responses can lead to pathological consequences, exacerbating disease progression.
Through their extensive research efforts, the Stevens Lab has pioneered our understanding of how these brain-resident immune cells can be both protectors and aggressors. Understanding this dichotomy is fundamental in developing therapies aimed at enhancing microglial functions in a way that benefits neuronal health. Targeting these immune pathways may shift the paradigm in how Alzheimer’s treatment approaches react to the disease, leading to novel strategies focused on rehabilitating the brain’s immune defenses.
The Impact of Basic Science on Alzheimer’s Treatment Advancements
Basic science research serves as the cornerstone for breakthroughs in Alzheimer’s treatment. Beth Stevens emphasizes the necessity of foundational studies that may initially seem distant from practical applications. By exploring cellular mechanisms and interactions at the microscopic level, researchers lay the groundwork for understanding how diseases like Alzheimer’s develop. This foundational understanding is essential to inform future therapeutic approaches that could fundamentally change the landscape of treatment options available for patients.
With advancements in our knowledge of microglial functions and their relation to neurodegenerative diseases, techniques derived from basic research can inform clinical practice. As a result, effective treatments that target the underlying processes driving Alzheimer’s are becoming increasingly tangible. By fostering an environment that encourages curiosity-driven research, organizations and funding bodies can facilitate pathways leading to novel interventions that stem directly from these essential scientific insights.
The Future of Neuroinflammation Research in Alzheimer’s
Research into neuroinflammation has gained momentum as a focal point in Alzheimer’s studies. Recognizing how microglial cells contribute to inflammation provides insight into the disease’s progression and potential treatment avenues. Stevens and her colleagues investigate the role of neuroinflammatory processes in the pathogenesis of Alzheimer’s, advocating for targeted therapies that could mitigate the detrimental effects of inflammation in the brain.
As scientists delve deeper into the complexities of neuroinflammatory signaling, they uncover how these pathways interact with other cellular mechanisms in the brain. Future studies will aim to elucidate the intricate networks at play, paving the way for comprehensive strategies that do not just manage symptoms but attack the disease’s root causes. This will require a multi-faceted approach, integrating knowledge from immunology, neurology, and pharmacology to develop holistic solutions that improve the lives of those affected by Alzheimer’s.
Pioneering New Biomarkers for Early Alzheimer’s Detection
Early detection of Alzheimer’s disease is crucial for effective intervention and potential treatment success. New biomarkers identified through research in microglial cell behavior hold promise for providing earlier diagnostic tools. Beth Stevens’ studies contribute to the growing body of knowledge regarding the role of microglial dysfunction as an early indicator of neurodegeneration, laying the foundation for advancements in the diagnosis and management of Alzheimer’s.
Detecting Alzheimer’s at an earlier stage enables healthcare professionals to implement timely interventions that can slow the disease’s progression. Innovative biomarkers serve not only as diagnostic tools but may also play a role in stratifying patients for clinical trials, thereby enhancing the precision medicine approach. As these discoveries continue to evolve, they may lead to revolutionary changes in how Alzheimer’s disease is identified and treated.
Funding and Support for Alzheimer’s Research Initiatives
A significant challenge in advancing Alzheimer’s research is the need for sustained funding and support. Stevens acknowledges the critical role that federal funding from organizations such as the National Institutes of Health plays in fostering exploration of complex neurobiological phenomena. These resources empower researchers to pursue potentially groundbreaking work that could lead to significant improvements in understanding and treating neurodegenerative diseases.
The prospect of an aging population amplifies the urgency for governmental and private funding in Alzheimer’s research. Without adequate investment, the potential for breakthrough discoveries diminishes, threatening the advancement of therapies that can mitigate the disease’s catastrophic impact. Advocating for continued and increased funding for Alzheimer’s research is essential in ensuring that scientists can continue to explore the interactions between brain immune responses and neurodegeneration.
The Interconnectedness of Neural Development and Alzheimer’s Disease
Understanding the interaction between neural development and Alzheimer’s disease is paramount in the quest for treatment. Microglial cells, pivotal during developmental stages, have been shown to continue influencing neuronal health throughout life. Beth Stevens’ work highlights how disruptions in microglial activity during key growth phases can predispose individuals to neurodegenerative diseases later on, underscoring a biological narrative that spans an individual’s life.
Addressing these intersections opens new avenues for intervention strategies that focus not only on older adults but also on identifying potential risk factors earlier in life. By targeting mechanisms at the intersection of development and disease, researchers can craft holistic preventive strategies that may reduce the incidence of Alzheimer’s and similar disorders, ultimately benefiting public health at large.
Collaborative Approaches to Alzheimer’s Research
Collaboration is a vital element in Alzheimer’s research, bringing together diverse expertise to tackle complex challenges. The Stevens Lab’s partnership with various institutions serves as an exemplary model for collaborative science, facilitating holistic exploration of neurodegenerative diseases. Such teamwork enhances knowledge sharing, accelerating the translation of basic research findings into clinical settings, thus benefiting Alzheimer’s treatment efforts.
Interdisciplinary collaborations allow for integrating insights from different fields, enhancing the understanding of brain immune systems, microglial functions, and their implications for Alzheimer’s. As researchers align around shared goals, the potential for innovative solutions increases, making collective efforts a cornerstone in the ongoing fight against Alzheimer’s disease and ensuring that progress is made toward successful therapeutic interventions.
The Importance of Patient-Centric Research in Alzheimer’s
Patient-centric research emphasizes the importance of understanding Alzheimer’s from the perspective of those affected. By prioritizing patient experiences and outcomes, researchers can tailor studies to address real-world challenges faced by individuals with Alzheimer’s and their families. Beth Stevens champions this approach, ensuring that discoveries made in the laboratory reflect the needs and concerns of patients, ultimately guiding the development of more effective treatment protocols.
Including patient perspectives helps refine research questions, aligning scientific exploration with the realities of neurological disorders. As Alzheimer’s research progresses with this patient-centric focus, it can lead to more relevant and applicable treatments that resonate within the community of those impacted by the disease, fostering a deeper connection between science and individual experiences.
Frequently Asked Questions
How do microglial cells relate to Alzheimer’s research?
Microglial cells are crucial to Alzheimer’s research as they function as the brain’s immune system. They are responsible for maintaining brain health by eliminating dead cells and pruning synapses. Dysregulation of this pruning process is linked to neurodegenerative diseases such as Alzheimer’s, making microglia a focal point for developing new treatments.
What is Beth Stevens’ contribution to Alzheimer’s treatment and research?
Beth Stevens has significantly advanced Alzheimer’s treatment research by revealing how microglial cells impact synaptic pruning and brain health. Her findings have implications for understanding Alzheimer’s disease and have opened avenues for new therapeutic approaches and early diagnostic biomarkers.
Why are microglial cells important in the study of neurodegenerative diseases like Alzheimer’s?
Microglial cells are important in neurodegenerative diseases such as Alzheimer’s because they are involved in the brain’s immune response. Their improper function can lead to neuronal damage and contribute to the progression of Alzheimer’s, making them critical targets for potential treatments.
What role does the brain’s immune system play in Alzheimer’s disease?
The brain’s immune system, primarily mediated by microglial cells, plays a vital role in Alzheimer’s disease. These cells monitor brain health, respond to damage, and influence neuronal connectivity. Research shows that their dysregulation is linked to disease pathology, highlighting their importance in Alzheimer’s research.
How does Beth Stevens’ research impact future Alzheimer’s treatment options?
Beth Stevens’ research has paved the way for innovative Alzheimer’s treatment options by identifying the role of microglial cells in synaptic pruning and neuroinflammation. Her discoveries may help develop medications that enhance microglial function or address underlying disease mechanisms, ultimately improving patient outcomes.
What are the implications of Stevens’ findings on Alzheimer’s biomarkers?
Stevens’ findings contribute to identifying new biomarkers for Alzheimer’s disease, which can lead to earlier detection. By understanding how microglial cells operate and their role in neurodegenerative disorders, researchers can develop more effective diagnostic tools and interventions.
How does Alzheimer’s research involving microglial cells evolve over time?
Alzheimer’s research involving microglial cells evolves as new discoveries are made about their functions in brain health. Ongoing studies continue to uncover how these cells interact with neurons and contribute to disease pathology, which informs the development of novel Alzheimer’s treatment strategies.
What is the significance of funding in Beth Stevens’ Alzheimer’s research?
Funding, especially from institutions like the National Institutes of Health, has been crucial for Beth Stevens’ Alzheimer’s research. It supports foundational studies necessary for understanding microglial function and exploring their role in neurodegenerative diseases, ultimately aiding the quest for effective treatments.
| Key Point | Details |
|---|---|
| Beth Stevens’ Research | Focuses on microglial cells and their role in the brain’s immune system, contributing to our understanding of neurodegenerative diseases like Alzheimer’s. |
| Role of Microglia | Microglia eliminate dead cells and prune synapses, but improper function can lead to diseases including Alzheimer’s. |
| Impact of Research | Stevens’ findings may lead to new medications and identified biomarkers for early disease detection. |
| Future of Alzheimer’s Treatment | The growing elderly population may increase the incidence of Alzheimer’s, with significant implications for care costs in the future. |
| Funding Importance | Federal funding has been vital to pursue and advance research in neurodegenerative diseases. |
Summary
Alzheimer’s research continues to progress through crucial discoveries made by dedicated scientists like Beth Stevens. By advancing our knowledge of microglial cells and their role in brain health, Stevens is helping to pave the way for innovative treatments and earlier detection of Alzheimer’s disease. This vital research is essential, considering the anticipated doubling of Alzheimer’s cases by 2050 and the associated welfare costs. With ongoing exploration supported by federal funding, the hope for effective therapies grows stronger, enhancing the quality of life for millions affected by this devastating disease.